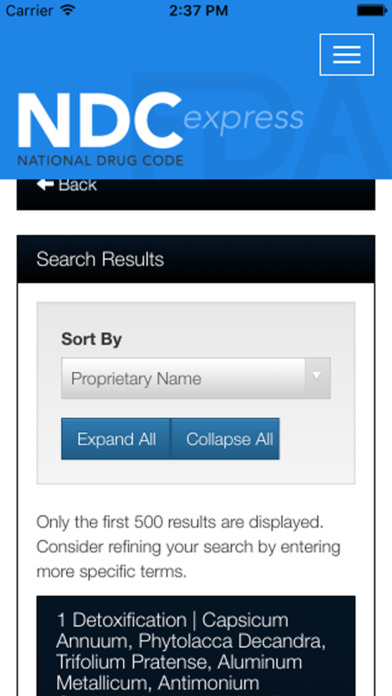
Currently, drug products are identified and reported using a unique, 10-digit, three-segment number. This is called the National Drug Code, most often referred to as the NDC number. The FDA assigns the first segment, the labeler code. A “labeler” is a firm that manufactures or distributes the drug, including drug re-packagers or re-labelers. This segment contains 4 or 5 digits. The second segment, the product code, identifies the drug, including the specific strength, dosage form, and formulation. The third segment, the package code, identifies package sizes and types. The firm assigns the product and package codes.
All drugs entering U.S. commercial distribution are required to be listed with the FDA. Firms submit the NDCs numbers assigned to the drugs they manufacture or distribute as part of their listing requirements. The FDA publishes these NDC numbers and other information submitted the online NDC Directory The assignment of an NDC number does not in any way denote FDA approval of the product.